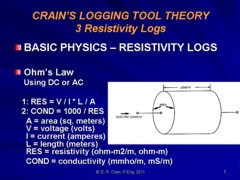
|
Group → |
1 |
2 |
3 |
|
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
| |
Alkali metal |
Alkaline
earth metal |
|
|
|
|
|
|
|
|
|
|
|
Boron group |
Carbon group |
Pnictogen |
Chalcogen |
Halogen |
Noble gas |
|
CAS: |
IA |
IIA |
IIIB |
|
IVB |
VB |
VIB |
VIIB |
VIIIB |
IB |
IIB |
IIIA |
IVA |
VA |
VIA |
VIIA |
VIIIA |
|
old IUPAC: |
IA |
IIA |
IIIA |
|
IVA |
VA |
VIA |
VIIA |
VIII |
IB |
IIB |
IIIB |
IVB |
VB |
VIB |
VIIB |
0 |
|
Period ↓
Period ↓ |
Hydrogen
-
[7000100780000000000♠1.0078, 7000100820000000000♠1.0082]
- short: 7000100800000000000♠1.008
|
→element
name
atomic number
chemical symbol
|
|
Helium
-
7000400260200000000♠4.002602(2)
- short:
7000400260000000000♠4.0026
|
|
2 |
Lithium
-
[7000693800000000000♠6.938, 7000699700000000000♠6.997]
- short: 7000694000000000000♠6.94
|
Beryllium
-
7000901218310000000♠9.0121831(5)
- short:
7000901220000000000♠9.0122
|
|
Boron
-
[7001108059999999999♠10.806, 7001108210000000000♠10.821]
- short: 7001108100000000000♠10.81
|
Carbon
-
[7001120090000000000♠12.009, 7001120120000000000♠12.012]
- short: 7001120110000000000♠12.011
|
Nitrogen
-
[7001140060000000000♠14.006, 7001140079999999999♠14.008]
- short: 7001140070000000000♠14.007
|
Oxygen
-
[7001159990000000000♠15.999, 7001160000000000000♠16.000]
- short: 7001159990000000000♠15.999
|
Fluorine
-
7001189984031630000♠18.998403163(6)
- short:
7001189980000000000♠18.998
|
Neon
-
7001201797000000000♠20.1797(6)
- short:
7001201800000000000♠20.180
|
|
3 |
Sodium
-
7001229897692800000♠22.98976928(2)
- short:
7001229899999999999♠22.990
|
Magnesium
-
[7001243040000000000♠24.304, 7001243070000000000♠24.307]
- short: 7001243050000000000♠24.305
|
|
Aluminium
-
7001269815385000000♠26.9815385(7)
- short:
7001269820000000000♠26.982
|
Silicon
-
[7001280840000000000♠28.084, 7001280860000000000♠28.086]
- short: 7001280850000000000♠28.085
|
Phosphorus
-
7001309737619980000♠30.973761998(5)
- short:
7001309740000000000♠30.974
|
Sulfur
-
[7001320590000000000♠32.059, 7001320760000000000♠32.076]
- short: 7001320600000000000♠32.06
|
Chlorine
-
[7001354460000000000♠35.446, 7001354570000000000♠35.457]
- short: 7001354500000000000♠35.45
|
Argon
-
7001399480000000000♠39.948(1)
- short:
7001399480000000000♠39.948
|
|
4 |
Potassium
-
7001390983000000000♠39.0983(1)
- short:
7001390980000000000♠39.098
|
Calcium
-
7001400780000000000♠40.078(4)
- short:
7001400780000000000♠40.078(4)
|
Scandium
-
7001449559080000000♠44.955908(5)
- short:
7001449560000000000♠44.956
|
|
Titanium
-
7001478670000000000♠47.867(1)
- short:
7001478670000000000♠47.867
|
Vanadium
-
7001509415000000000♠50.9415(1)
- short:
7001509420000000000♠50.942
|
Chromium
-
7001519961000000000♠51.9961(6)
- short:
7001519960000000000♠51.996
|
Manganese
-
7001549380440000000♠54.938044(3)
- short:
7001549380000000000♠54.938
|
Iron
-
7001558450000000000♠55.845(2)
- short:
7001558450000000000♠55.845(2)
|
Cobalt
-
7001589331940000000♠58.933194(4)
- short:
7001589330000000000♠58.933
|
Nickel
-
7001586934000000000♠58.6934(4)
- short:
7001586930000000000♠58.693
|
Copper
-
7001635460000000000♠63.546(3)
- short:
7001635460000000000♠63.546(3)
|
Zinc
-
7001653800000000000♠65.38(2)
- short:
7001653800000000000♠65.38(2)
|
Gallium
-
7001697230000000000♠69.723(1)
- short:
7001697230000000000♠69.723
|
Germanium
-
7001726300000000000♠72.630(8)
- short:
7001726300000000000♠72.630(8)
|
Arsenic
-
7001749215950000000♠74.921595(6)
- short:
7001749220000000000♠74.922
|
Selenium
-
7001789710000000000♠78.971(8)
- short:
7001789710000000000♠78.971(8)
|
Bromine
-
[7001799010000000000♠79.901, 7001799070000000000♠79.907]
- short: 7001799040000000000♠79.904
|
Krypton
-
7001837980000000000♠83.798(2)
- short:
7001837980000000000♠83.798(2)
|
|
5 |
Rubidium
-
7001854678000000000♠85.4678(3)
- short:
7001854680000000000♠85.468
|
Strontium
-
7001876200000000000♠87.62(1)
- short:
7001876200000000000♠87.62
|
Yttrium
-
7001889058400000000♠88.90584(2)
- short:
7001889060000000000♠88.906
|
|
Zirconium
-
7001912240000000000♠91.224(2)
- short:
7001912240000000000♠91.224(2)
|
Niobium
-
7001929063700000000♠92.90637(2)
- short:
7001929060000000000♠92.906
|
Molybdenum
-
7001959500000000000♠95.95(1)
- short:
7001959500000000000♠95.95
|
Technetium
[98]
|
Ruthenium
-
7002101070000000000♠101.07(2)
- short:
7002101070000000000♠101.07(2)
|
Rhodium
-
7002102905500000000♠102.90550(2)
- short:
7002102910000000000♠102.91
|
Palladium
-
7002106420000000000♠106.42(1)
- short:
7002106420000000000♠106.42
|
Silver
-
7002107868200000000♠107.8682(2)
- short:
7002107870000000000♠107.87
|
Cadmium
-
7002112414000000000♠112.414(4)
- short:
7002112410000000000♠112.41
|
Indium
-
7002114818000000000♠114.818(1)
- short:
7002114820000000000♠114.82
|
Tin
-
7002118710000000000♠118.710(7)
- short:
7002118710000000000♠118.71
|
Antimony
-
7002121760000000000♠121.760(1)
- short:
7002121760000000000♠121.76
|
Tellurium
-
7002127600000000000♠127.60(3)
- short:
7002127600000000000♠127.60(3)
|
Iodine
-
7002126904470000000♠126.90447(3)
- short:
7002126900000000000♠126.90
|
Xenon
-
7002131293000000000♠131.293(6)
- short:
7002131289999999999♠131.29
|
|
6 |
Caesium
-
7002132905451960000♠132.90545196(6)
- short:
7002132910000000000♠132.91
|
Barium
-
7002137327000000000♠137.327(7)
- short:
7002137330000000000♠137.33
|
Lanthanum
-
7002138905470000000♠138.90547(7)
- short:
7002138910000000000♠138.91
|
58–71
 |
Hafnium
-
7002178490000000000♠178.49(2)
- short:
7002178490000000000♠178.49(2)
|
Tantalum
-
7002180947880000000♠180.94788(2)
- short:
7002180950000000000♠180.95
|
Tungsten
-
7002183840000000000♠183.84(1)
- short:
7002183840000000000♠183.84
|
Rhenium
-
7002186207000000000♠186.207(1)
- short:
7002186210000000000♠186.21
|
Osmium
-
7002190230000000000♠190.23(3)
- short:
7002190230000000000♠190.23(3)
|
Iridium
-
7002192217000000000♠192.217(3)
- short:
7002192220000000000♠192.22
|
Platinum
-
7002195084000000000♠195.084(9)
- short:
7002195080000000000♠195.08
|
Gold
-
7002196966569000000♠196.966569(5)
- short:
7002196970000000000♠196.97
|
Mercury
-
7002200592000000000♠200.592(3)
- short:
7002200590000000000♠200.59
|
Thallium
-
[7002204380000000000♠204.38, 7002204390000000000♠204.39]
- short: 7002204380000000000♠204.38
|
Lead
-
7002207200000000000♠207.2(1)
- short:
7002207200000000000♠207.2
|
Bismuth
-
7002208980400000000♠208.98040(1)
- short:
7002208980000000000♠208.98
|
Polonium
[209]
|
Astatine
[210]
|
Radon
[222]
|
|
7 |
Francium
[223]
|
Radium
[226]
|
Actinium
[227]
|
90–103
.svg/20px-Asterisks_2_(vertical).svg.png) |
Rutherfordium
[267]
|
Dubnium
[268]
|
Seaborgium
[269]
|
Bohrium
[270]
|
Hassium
[270]
|
Meitnerium
[278]
|
Darmstadtium
[281]
|
Roentgenium
[282]
|
Copernicium
[285]
|
Nihonium
[286]
|
Flerovium
[289]
|
Moscovium
[290]
|
Livermorium
[293]
|
Tennessine
[294]
|
Oganesson
[294]
|
|
| |
 |
Cerium
-
7002140116000000000♠140.116(1)
- short:
7002140120000000000♠140.12
|
Praseodymium
-
7002140907660000000♠140.90766(2)
- short:
7002140910000000000♠140.91
|
Neodymium
-
7002144242000000000♠144.242(3)
- short:
7002144240000000000♠144.24
|
Promethium
[145]
|
Samarium
-
7002150360000000000♠150.36(2)
- short:
7002150360000000000♠150.36(2)
|
Europium
-
7002151964000000000♠151.964(1)
- short:
7002151960000000000♠151.96
|
Gadolinium
-
7002157250000000000♠157.25(3)
- short:
7002157250000000000♠157.25(3)
|
Terbium
-
7002158925350000000♠158.92535(2)
- short:
7002158930000000000♠158.93
|
Dysprosium
-
7002162500000000000♠162.500(1)
- short:
7002162500000000000♠162.50
|
Holmium
-
7002164930330000000♠164.93033(2)
- short:
7002164930000000000♠164.93
|
Erbium
-
7002167259000000000♠167.259(3)
- short:
7002167260000000000♠167.26
|
Thulium
-
7002168934220000000♠168.93422(2)
- short:
7002168930000000000♠168.93
|
Ytterbium
-
7002173045000000000♠173.045(10)
- short:
7002173050000000000♠173.05
|
Lutetium
-
7002174966800000000♠174.9668(1)
- short:
7002174970000000000♠174.97
|
| |
.svg/20px-Asterisks_2_(vertical).svg.png) |
Thorium
-
7002232037700000000♠232.0377(4)
- short:
7002232040000000000♠232.04
|
Protactinium
-
7002231035880000000♠231.03588(2)
- short:
7002231040000000000♠231.04
|
Uranium
-
7002238028910000000♠238.02891(3)
- short:
7002238030000000000♠238.03
|
Neptunium
[237]
|
Plutonium
[244]
|
Americium
[243]
|
Curium
[247]
|
Berkelium
[247]
|
Californium
[251]
|
Einsteinium
[252]
|
Fermium
[257]
|
Mendelevium
[258]
|
Nobelium
[259]
|
Lawrencium
[266]
|