|
 GEOCHEMICAL ANALYSIS BASICS
GEOCHEMICAL ANALYSIS BASICS
Geochemical analysis is used to determine the type and
quantity of organic carbon and other properties of
unconventional reservoirs and source rocks. Organic carbon
in the form of kerogen is the remnant of ancient life preserved in
sedimentary rocks, after degradation by bacterial and chemical
processes, and further modified by temperature, pressure, and time.
The latter step, called thermal maturation, is a function of burial
history (depth) and proximity to heat sources. Maturation provides
the chemical reactions needed to give us gas, oil, bitumen,
pyrobitumen, and graphite (pure carbon) that we find while drilling
wells for petroleum.
Organic
carbon is usually associated with shales or silty shales,
but may be present in relatively clean siltstone, sandstone, and carbonate rocks.
A source rock is a fine grained sediment
rich in organic matter that could generate crude oil or natural gas
after thermal alteration of kerogen in the Earth's crust. The oil or
gas could then migrate from the source rock to more porous and
permeable sediments, where ultimately the oil or gas could
accumulate to make a commercial oil or gas reservoir.
If a source rock has
not been exposed to temperatures of about 100 °C, it is termed a
potential source rock. If generation and expulsion of oil or gas
have occurred, it is termed an actual source rock. The terms
immature and mature are commonly used to describe source rocks and
also the current state of the kerogen contained in the rock.
Total organic
carbon (TOC) as measured by laboratory techniques historically has
been used to assess the quality of source rocks,
but now is widely used to help evaluate some unconventional reservoirs
(reservoirs that are both source and productive).
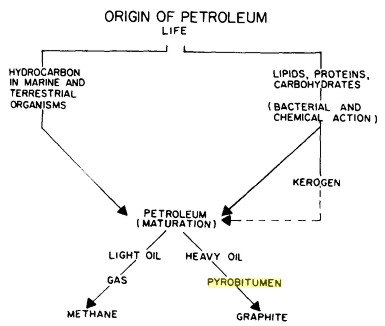  Pathways that
convert living organisms to organic carbon, from "Bitumens,
Asphalts, and Tar Sands" by
George V.
Chilingar,
Teh Fu Yen, 1978. Pathways that
convert living organisms to organic carbon, from "Bitumens,
Asphalts, and Tar Sands" by
George V.
Chilingar,
Teh Fu Yen, 1978.
In the lab, it is relatively easy to distinguish kerogen from hydrocarbons:
kerogen is insoluble in organic solvents, oil and bitumen are
soluble. Pyrobitumen is not soluble but its hardness is used to
identify it from kerogen.
Graphite is evident on resistivity logs because of the very
low resistivity; all other forms of organic carbon are resistive.
Organic
carbon has a density near that of water, so it looks like porosity
to conventional porosity logs. High resistivity with some apparent porosity on a log
analysis is a good indicator of organic carbon content OR
ordinary hydrocarbons OR both.
 TYPES OF KEROGEN
TYPES OF KEROGEN
Organic
material can be classified according to the source of
the material, as shown below.
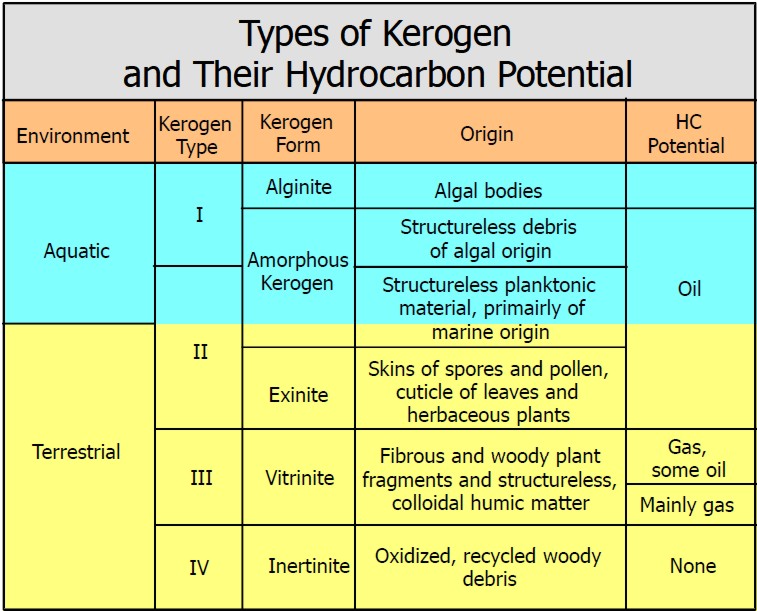
Origin, type, source, and
hydrocarbon potential of different kerogens.
Organic content in gas shales is usually Type II,
as opposed to coals, which contain mostly Type III
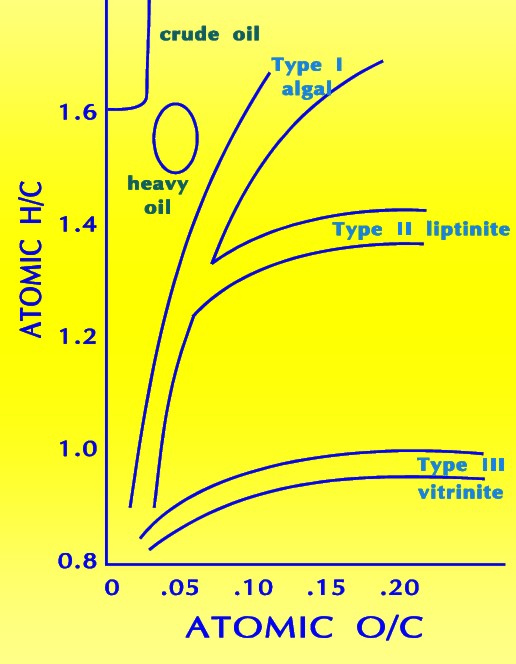 The
most commonly utilized scheme for classifying organic matter in
sediments is based on the relative abundance of elemental carbon,
oxygen, and hydrogen plotted graphically as the H/C and O/C ratio on
a so called Van Krevelen diagram. The
most commonly utilized scheme for classifying organic matter in
sediments is based on the relative abundance of elemental carbon,
oxygen, and hydrogen plotted graphically as the H/C and O/C ratio on
a so called Van Krevelen diagram.
The classic Van Krevelen diagram 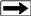
Rather than plot the elemental ratios it is common to plot indices
determined by a pyrolysis technique referred to as Rock Eval. In the
pyrolysis techniques two indices are determined: the Hydrogen Index
(HI) which is milligrams of pyrolyzable hydrocarbons divided by TOC
and the Oxygen Index (OI) which is milligrams of pyrolyzable organic
carbon dioxide divided by TOC.
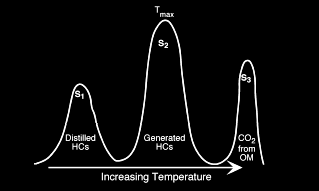
Cross-plots of both elemental H/C and O/C ratios or of HI and OI are
utilized to discriminate four ‘fields’ which are referred to as
Types I, II, III, and IV kerogen.
Type I kerogen is hydrogen rich (atomic H/C of 1.4 to 1.6: HI of >
700) and is derived predominantly from zooplankton, phytoplankton,
micro-organisms (mainly bacteria) and lipid rich components of
higher plants (H/C ratio 1.7 to 1.9).
Type II kerogen is intermediate in composition (H/C ≈ 1.2: HI ≈ 600)
and derived from mixtures of highly degraded and partly oxidized
remnants of higher plants or marine phytoplankton.
Type III kerogen is hydrogen poor (H/C ratio 1.3 to 1.5) and oxygen
rich and is mainly derived from cellulose and lignin derived from
higher plants.
Type IV kerogen is hydrogen poor and oxygen rich and essentially
inert. This organic matter is mainly derived from charcoal and
fungal bodies. Type IV kerogen is not always distinguished but is
grouped with Type III.
The different types of organic matter are of fundamental importance
since the relative abundance of hydrogen, carbon, and oxygen
determines what products can be generated from the organic matter
upon diagenesis. Since coal is comprised predominantly of Type III
kerogen, there is little liquid hydrogen generating capacity. If the
coal includes abundant hydrogen rich components (such as spores,
pollen, resin, waxes - Type I or II), it will generate some liquid
hydrocarbons. Although not common, some oil deposits are thought to
be sourced by coals.
Note: Portions of the above
Section, and the next Section, were taken verbatim (with moderate
editing) from CBM Solutions reports.
 Analyzing TOC IN THE LABORATORY
Analyzing TOC IN THE LABORATORY
The total
organic carbon content of rocks is obtained by heating the
rock in a furnace and combusting the organic matter to
carbon dioxide. The amount of carbon dioxide liberated is
proportional to the amount of carbon liberated in the
furnace, which in turn is related to the carbon content of
the rock. The carbon dioxide liberated can be measured
several different ways. The most common methods use a
thermal conductivity detector or infrared spectroscopy.
Many source rocks also include inorganic sources of carbon
such as carbonates and most notably calcite, dolomite, and
siderite. These minerals break down at high temperature,
generating carbon dioxide and thus their presence must be
corrected in order to determine the organic carbon content.
Generally, the amount of carbonate is determined by acid
digestion (normally 50% HCl) and the carbon dioxide
generated is measured and then subtracted from the total
carbon dioxide to obtain the organic fraction.
Total organic
carbon is often taken to mean the same thing as kerogen, but this is
not the case. Kerogen is made up of oxygen, nitrogen, sulphur, and
hydrogen, in addition to carbon. The standard pyrolysis lab
procedure measures only the carbon, so total organic carbon excludes
the other elements.
About 80% of a typical kerogen (by weight) is carbon, so the weight
fraction of TOC is 80% of the kerogen weight. The factor is
lower for less mature and higher for more mature kerogen:
1: Wtoc = Wker * KTOC
OR 2: Wker = Wtoc / KTOC
Where:
Wtoc = weight fraction of organic carbon
Wker = weight fraction of kerogen
KTOC = kerogen correction factor - range = 0.68 to 0.90, default 0.80
Another
lab procedure, called RockEval, burns both hydrogen and carbon, so
the data needs to be calibrated to the standard method by performing
a chemical analysis on the kerogen. Typically the organic carbon
needs to be reduced by about 10% (the weight of the hydrogen burned)
to match the standard method.
Rock Eval is the trade name for a set of equipment used in the lab
to measure organic content of rocks, as well as other properties of
the organics that help to identify the kerogen type. Rock-Eval combusts
a crushed sample of rock at 600ºC. Refractory organic matter
such as inertinite does not combust readily at 600ºC so most coal
samples yield Rock-Eval measured TOC values much lower than actual
values because of incomplete combustion. Rock-Eval is not
recommended for use with coals or source rocks with significant
amounts of Type III and IV kerogen.
A rock sample is crushed finely enough so that 85% falls through a
75 mesh screen. Approximately 100 mg of sample is loaded into a
stainless steel crucible capped with a micro mesh filter. To ensure
accuracy, standard samples are loaded at the beginning and end of
the run. Any drift in data can be detected and the samples rerun if
necessary.
The analyzer consists of a flame ionization detector and two IR
detector cells. The free hydrocarbons (S1) are determined from an
isothermal heating of the sample at 340 degrees Celsius. These
hydrocarbons are measured by the flame ionization detector. The
temperature is then increased from 340 to 640 degrees Celsius.
Hydrocarbons are then released from the kerogen and measured by the
flame ionization detector creating the S2 peak. The temperature at
which S2 reaches its maximum rate of hydrocarbon generation is
referred to as Tmax. The CO2 generated from the oxidation step in
the 340 to 580 degrees Celsius is measured by the IR cells and is
referred to the S3 peak.
 Measured results from a typical Rock Eval study will contain: Measured results from a typical Rock Eval study will contain:
TOC% - Weight percentage of organic carbon
S1 = amount of free hydrocarbons in sample (mg/g)
S2 = amount of hydrocarbons generated through thermal
cracking (mg/g) –
provides the quantity of
hydrocarbons that the
rock has the potential to
produce through diagenesis.
S3 = amount of CO2 (mg of CO2/g of rock) - reflects the amount of oxygen
in the oxidation step.
Ro = vitrinite reflectance (%)
Tmax = the temperature at which maximum rate of
generation
of hydrocarbons occurs.
Calculated results include:
Hydrogen index
1: HI = 100 * S2 / TOC%
Oxygen index
2: OI = 100 * S3 / TOC%
Production index
3: PI = S1 / (S1 + S2)
|
Depth (m) |
TOC |
SRA |
Tmax |
Meas. |
HI |
OI |
S2/S3 |
S1/TOC*100 |
PI |
|
Top |
S1 |
S2 |
S3 |
(°C) |
% Ro |
|
X025 |
1.35 |
0.05 |
1.72 |
0.63 |
444 |
|
128 |
47 |
3 |
4 |
0.03 |
|
X040 |
1.18 |
0.05 |
1.65 |
0.57 |
443 |
|
140 |
49 |
3 |
4 |
0.03 |
|
X050 |
0.83 |
0.03 |
1.31 |
0.55 |
443 |
|
158 |
66 |
2 |
4 |
0.02 |
|
X065 |
0.80 |
0.04 |
1.00 |
0.58 |
440 |
|
126 |
73 |
2 |
5 |
0.04 |
|
X075 |
0.75 |
0.05 |
1.04 |
0.72 |
438 |
|
138 |
96 |
1 |
7 |
0.05 |
|
X090 |
1.04 |
0.09 |
2.52 |
0.29 |
452 |
|
241 |
28 |
9 |
9 |
0.03 |
|
X110 |
1.02 |
0.05 |
1.16 |
0.56 |
441 |
|
114 |
55 |
2 |
5 |
0.04 |
|
X135 |
1.05 |
0.05 |
1.32 |
0.57 |
443 |
|
125 |
54 |
2 |
5 |
0.04 |
Laboratory measured TOC values (weight %) with measured and
computed indices
HI versus OI plot example, indicating Type III kerogen
An alternate
method for measuring TOC by solution rather than pyrolysis is
described below, from a 1980's TOC report from Australia.
"The samples are
analyzed for total organic carbon (TOC) according to AS 1038 Part 6.
Moisture determinations are made to permit conversion to a dry
basis. Carbon occurring as carbonate ion is determined to correct
the gross carbon data to give the organic carbon content. This is
done by driving off carbonate minerals with HCl acid.
The crushed and sieved (100 mesh) samples are weighed and
exhaustively extracted in a Soxhlet apparatus using a
benzene-methanol mixture. After removal of methanol by azeotropic
distillation with benzene, the residue in benzene is diluted with
hexane and the hydrocarbon solution separated by filtration from the
brown precipitate. The latter is then dissolved in methanol. The
yield of methanol soluble material is determined gravimetrically.
The hexane soluble portion of the extractable organic matter
(E.O.M.) is weighed and chromatographed on silica. Elution with
hexane gives predominantly alkanes and subsequent elution with
hexane/benzene yields mainly monocyclic and polycycllc aromatic
hydrocarbons. The eluted hydrocarbons are weighed, and then analyzed
by gas chromatography / mass spectrometry."
 Geochemical Logs
Geochemical Logs
Measured and calculated indices can be plotted versus depth; the
resulting log
is called a Geochemical Log.
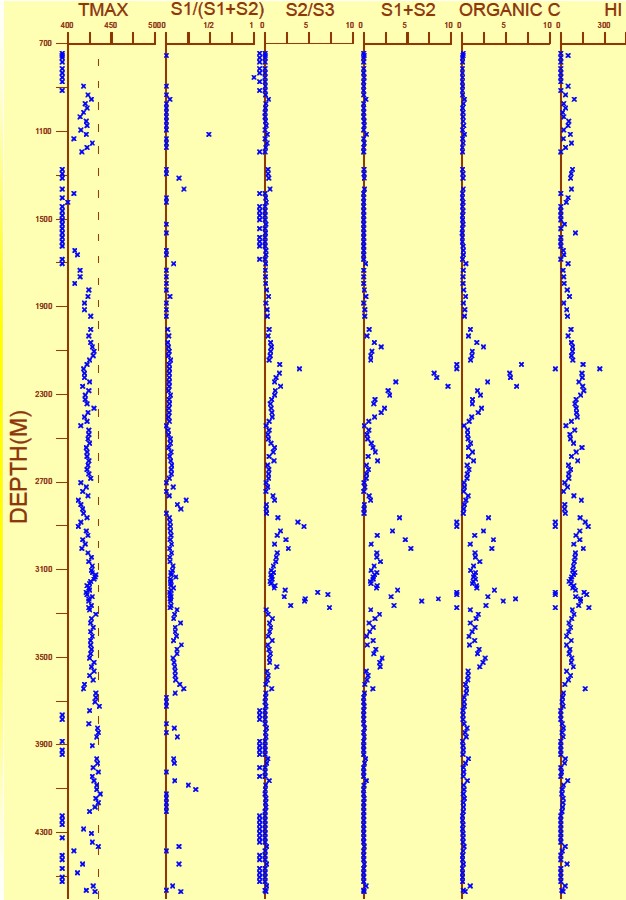
A geochemical log from offshore
East Coast Canada
 KEROGEN maturity
KEROGEN maturity
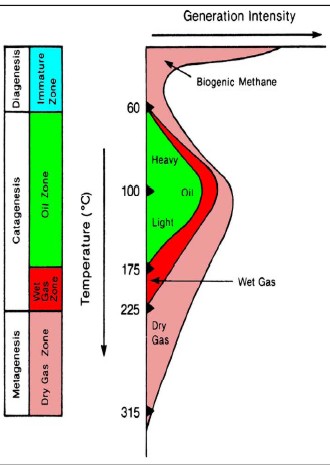 The
hydrocarbon potential of organic carbon depends on the thermal
history of the rocks containing the kerogen. Both temperature and
the time at that temperature determine the outcome. Medium
temperatures
(< 175 C) produce mostly oil and a little gas. Warmer temperatures
produce mostly gas. The
hydrocarbon potential of organic carbon depends on the thermal
history of the rocks containing the kerogen. Both temperature and
the time at that temperature determine the outcome. Medium
temperatures
(< 175 C) produce mostly oil and a little gas. Warmer temperatures
produce mostly gas.
Hydrocarbon
type versus temperature
defines "oil window" and "gas window",
with some obvious overlap 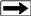
Vitrinite reflectance (Ro) is used as an indicator of the level of
organic maturity (LOM). Ro values between 0.60 and 0.78 usually
represent oil prone intervals. Ro > 0.78 usually indicates gas
prone. High values can suggest "sweet spots" for completing gas
shale wells.
Measurement of vitrinite reflectance was
described as follows from the 1980's TOC report.
"Sample
chips or sidewall core samples are cleaned to remove drilling mud or
mud cake and then crushed using a mortar and pestle to a grain-size
of less than 3 mm. Samples are mounted in cold-setting resin and
polished ''as received", so that whole-rock samples rather than
concentrates of organic matter are examined. This method is
preferred to the use of demineralized concentrates because of the
greater ease of identifying first generation vitrinite and, for
cuttings samples, of recognizing cavings. The core samples are
mounted and sectioned perpendicular to the bedd1ng.
Vitrinite reflectance measurements are made using immersion oil of
refractive index 1.518 at 546 nm and 23°C and spinel and garnet
standards of 0.42%, 0.917% and 1.726% reflectance for calibration.
Fluorescence-mode observations are made on all samples and provide
supplementary evidence concerning organic matter type, and exinite
abundance and maturity. For fluorescence-mode a 3 mm BG-3
excitation filter is used with a TK400 dichroic mirror and a K490
barrier filter."
Tmax is also a useful indicator of
maturity, higher values being more mature.
Graphs of HI vs Ro and HI vs Tmax are
used to help refine kerogen type and to assess maturity with respect
to the oil and gas "windows". Depth plots of Ro and Tmax are helpful
in spotting the top of the oil or gas window in specific wells, and
in locating sweet spots for possible production using horizontal
wells.
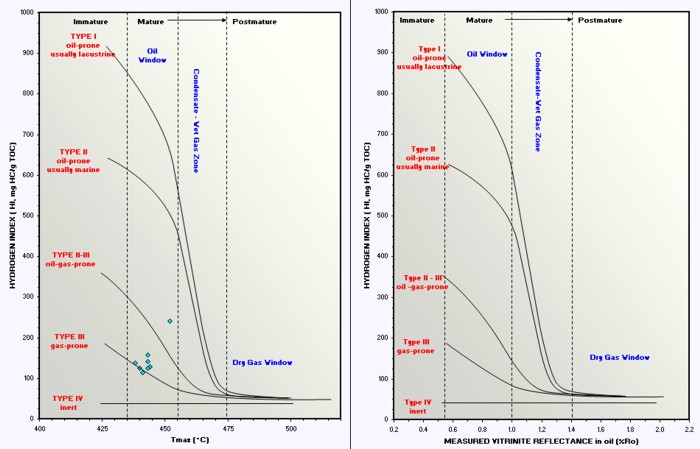
Crossplots of HI vs Tmax and HI vs Ro
determine organic maturity, kerogen type, and whether the rock is in
the oil or gas window. Immature and post mature rocks are not overly
interesting as possible source or reservoir rocks.
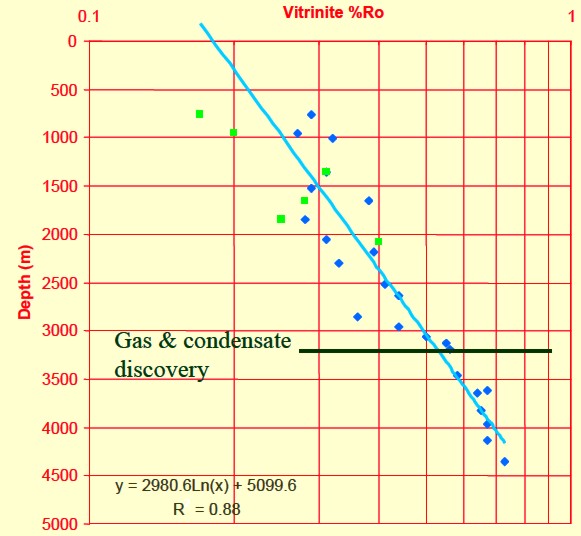
Depth plot of Ro to determine trend line and location of oil and gas
windows (Ro > 0.55).
Ro is plotted on a logarithmic scale, which makes the trend line
relatively straight.
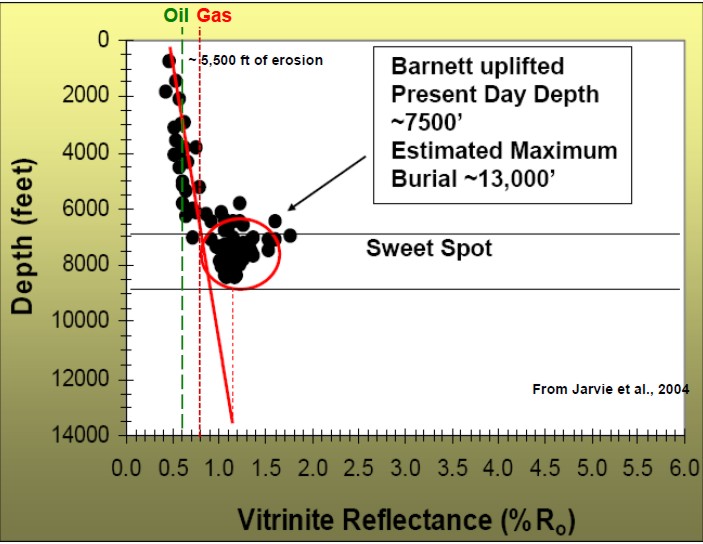
Thermal maturity as indicated by
vitrinite reflectance (Ro) versus depth for a Barnett shale, showing
"sweet spot" and
oil versus gas “windows”.
|

